Malaria Treatment for Newborns and Infants Approved for Use
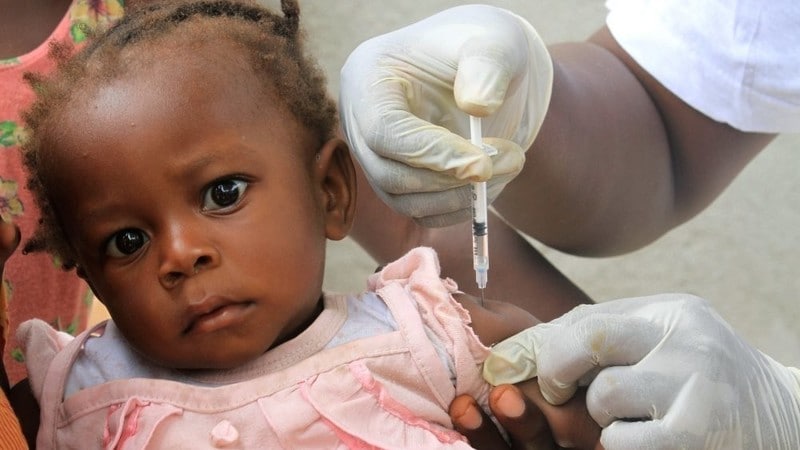
In a significant breakthrough in the fight against malaria, the first-ever medication specifically designed to treat malaria in newborns and very young infants has been officially approved for use. This development marks a historic milestone in global public health, as it directly addresses a long-standing treatment gap for the most vulnerable age group affected by the disease.
The newly approved medicine, created by pharmaceutical company Novartis, has received regulatory clearance from Swiss health authorities. Its deployment is expected to begin within a matter of weeks, particularly across African nations where malaria remains a major health crisis. The company has committed to making the drug available on a largely not-for-profit basis, aiming to ensure broad access in low-resource settings.
Until now, babies and small infants under 4.5 kilograms (approximately 10 pounds) had no malaria treatment formulated specifically for their needs. Instead, healthcare providers were forced to adapt medicines designed for older children. This practice often came with the risk of incorrect dosing, as the liver function and metabolic processes of infants are not yet fully developed. Such dosing inaccuracies could lead to overdoses or reduced effectiveness, posing a serious health threat.
This lack of age-appropriate treatment options has been referred to by experts as a dangerous “treatment gap,” particularly troubling given that the majority of malaria-related deaths occur in children under the age of five. According to the latest data from 2023, malaria was linked to approximately 597,000 deaths worldwide, with Africa bearing the brunt of this burden. Around 75% of these deaths were among children under five years old.
Health officials and child welfare advocates have welcomed the approval of this infant-specific treatment, viewing it as a critical step toward reducing malaria mortality in the youngest and most at-risk patients. The introduction of this tailored medication could potentially save hundreds of thousands of lives over time and reshape malaria care standards for infants globally.
As the medicine begins its phased rollout, it is expected to play a transformative role in malaria treatment, especially in areas with high transmission rates and limited healthcare infrastructure.





